A New Treatment
U.S. FDA Approves New Treatment for Highly Drug-Resistant TB
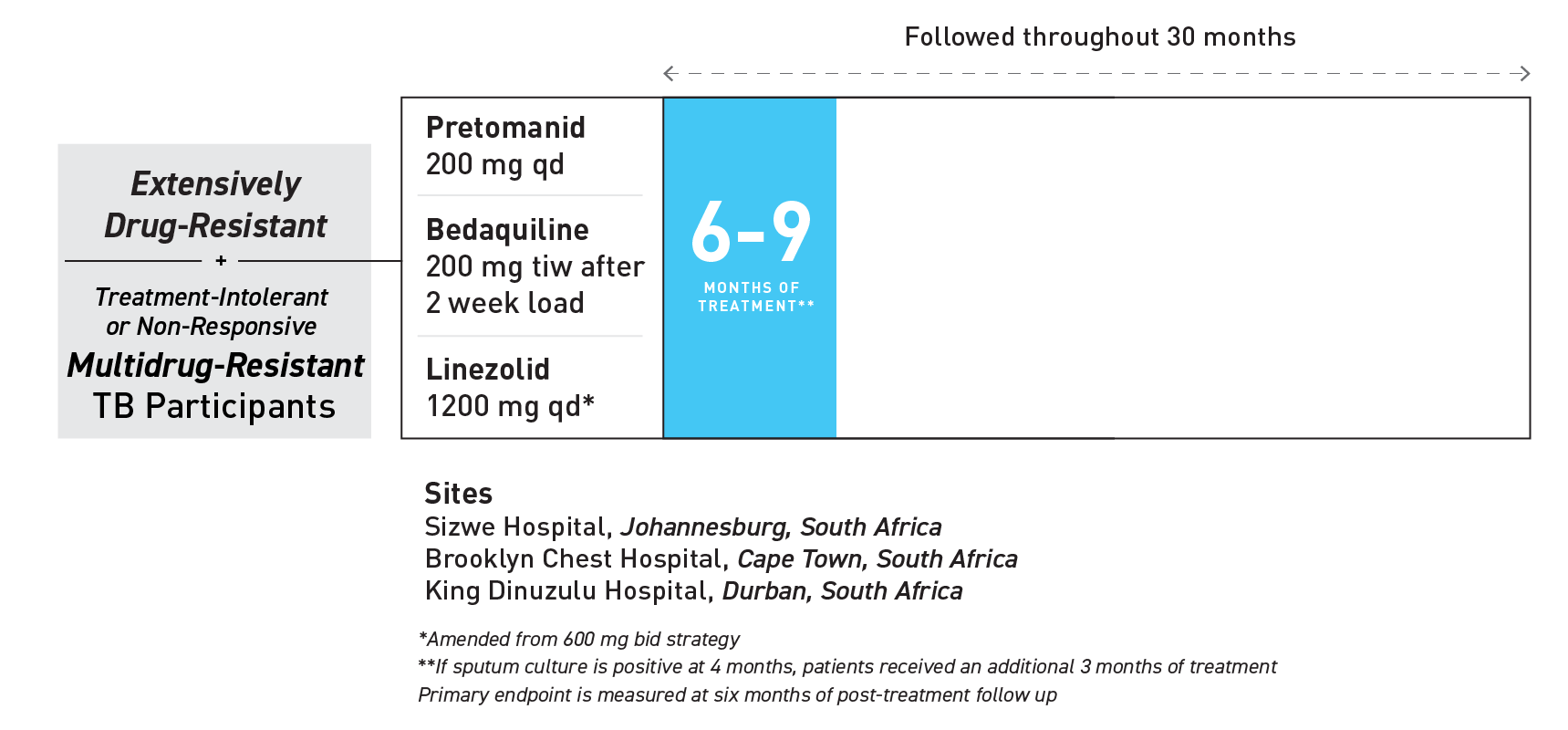
On August 14, 2019, TB Alliance received approval from the U.S. Food and Drug Administration (FDA) for its anti-TB drug pretomanid in a combination regimen for the treatment of people with highly drug-resistant forms of tuberculosis (TB).1 The approved indication is for the use of pretomanid as part of a three-drug regimen with bedaquiline and linezolid — collectively referred to as the BPaL regimen. The new drug was approved under the Limited Population Pathway for Antibacterial and Antifungal Drugs (LPAD pathway) as part of a six-month, all-oral regimen for the treatment of people with extensively drug-resistant TB (XDR-TB) or multidrug-resistant TB (MDR-TB) who are treatment-intolerant or non-responsive.1
Pretomanid is only the third new anti-TB drug approved for use by FDA in more than 40 years, as well as the first to be developed and registered by a not-for-profit organization.2,3
Licensed by TB Alliance in 2002, pretomanid has been evaluated in over 19 clinical trials enrolling more than 1,100 participants.1 It continues to be studied by TB Alliance and its research partners as part of BPaL and other pretomanid-containing regimens, including the ZeNix trial, which completed enrollment at the end of 2019.
Please see Full Prescribing Information at tballiance.org/pretomanid
Artist's rendering of the pretomanid compound
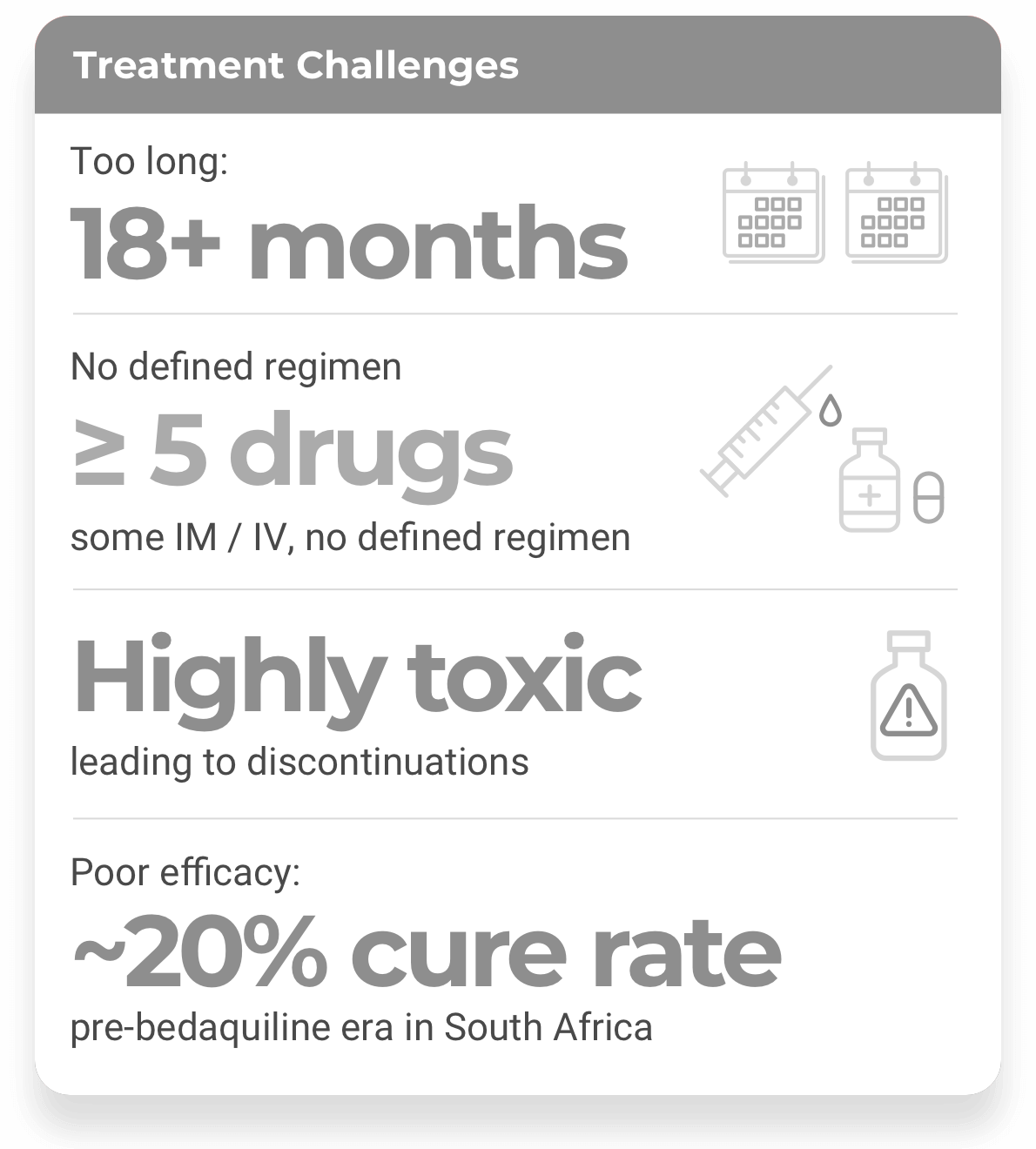
Historic XDR-TB Treatment vs. BPaL Regimen
Nix-TB: Phase 3 Clinical Trial
BPaL was studied in the pivotal Nix-TB clinical trial across three sites in South Africa.1 The trial enrolled 109 people with XDR-TB, as well as treatment-intolerant or non-responsive MDR-TB. As documented in the New England Journal of Medicine, Nix-TB data have demonstrated a successful outcome in 90 percent of patients who were treated with the BPaL regimen.4
“Until very recently, people infected with highly drug-resistant TB had poor treatment options and a poor prognosis.”
— Francesca Conradie, Principal Investigator, Nix-TB
Tsholofelo Msimango beats TB

Five years later, Ms. Msimango, 25, is now tuberculosis free. She is healthy and has a young son.
Tsholofelo participated in the Nix-TB clinical trial of the BPaL regimen. Read more of her story in The New York Times.

Ensuring Access Around the World
TB Alliance announced licensing agreements with multiple manufacturers to secure an affordable and sustainable market for pretomanid as part of the BPaL regimen. In April 2019, TB Alliance and Mylan announced a global commercialization partnership, giving Mylan the license to commercialize pretomanid for use in two regimens (BPaL and BPaMZ) to treat pulmonary TB. In October 2019, TB Alliance and Macleods announced a nonexclusive agreement to manufacture and sell pretomanid as part of the BPaL regimen in low- and middle-income countries. In December, Hongqi pharmaceuticals became the commercial partner for pretomanid as part of BPaL in China, Hong Kong, Macau and Taiwan.
In October, the Stop TB Partnership's Global Drug Facility (GDF) added pretomanid to its catalog of TB medicines. The "global access price" of US $364 for a six-month treatment course will be available to 150 countries representing the vast majority of the global TB burden.
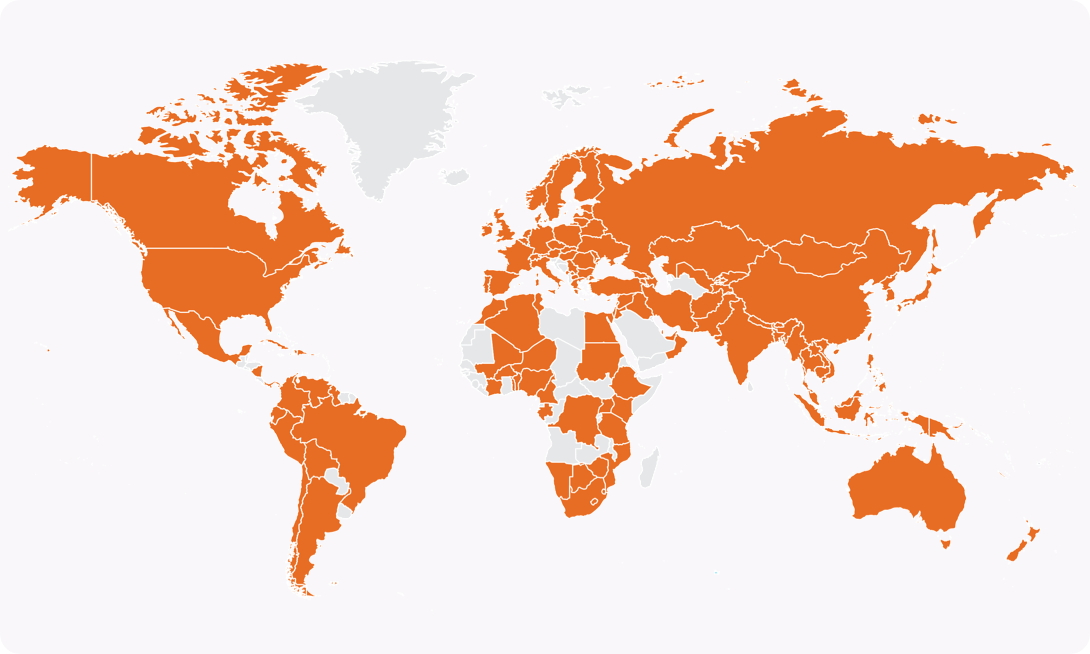
XDR-TB has been reported in 131 countries around the world
INDICATION | LIMITED POPULATION
Pretomanid Tablet is an antimycobacterial indicated, as part of a combination regimen with bedaquiline and linezolid for the treatment of adults with pulmonary extensively drug-resistant (XDR), treatment-intolerant or non-responsive multidrug-resistant (MDR) tuberculosis (TB). Approval of this indication is based on limited clinical safety and efficacy data. This drug is indicated for use in a limited and specific population of patients.
Limitations of Use:- Pretomanid Tablets are not indicated for patients with:
- Drug-sensitive (DS) tuberculosis
- Latent infection due to Mycobacterium tuberculosis
- Extra-pulmonary infection due to Mycobacterium tuberculosis
- MDR-TB that is not treatment-intolerant or non-responsive to standard therapy
- Safety and effectiveness of Pretomanid Tablets have not been established for its use in combination with drugs other than bedaquiline and linezolid as part of the recommended dosing regimen.
Contraindications
Pretomanid Tablets used in combination with bedaquiline and linezolid are contraindicated in patients for whom bedaquiline and/or linezolid is contraindicated.
Warnings and Precautions- Hepatic adverse reactions were reported with the use of the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid. Monitor symptoms and signs and liver related laboratory tests. Interrupt treatment with the entire regimen if evidence of liver injury occurs.
- Myelosuppression was reported with the use of the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid. Monitor complete blood counts. Decrease or interrupt linezolid dosing if significant myelosuppression develops or worsens.
- Peripheral and optic neuropathy were reported with the use of the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid. Monitor visual function. Obtain an ophthalmologic evaluation if there are symptoms of visual impairment. Decrease or interrupt linezolid dosing if neuropathy develops or worsens.
- QT prolongation was reported with the use of the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid. Use with drugs that prolong the QT interval may cause additive QT prolongation. Monitor ECGs. Discontinue the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid if significant ventricular arrhythmia or if QTcF interval prolongation of greater than 500 ms develops.
- Reproductive effects: Pretomanid caused testicular atrophy and impaired fertility in male rats. Advise patients of reproductive toxicities seen in animal studies and that the potential effects on human male fertility have not been adequately evaluated.
- Lactic acidosis was reported with the use of the combination regimen of Pretomanid Tablets, bedaquiline, and linezolid. Consider interrupting linezolid or the entire combination regimen of Pretomanid Tablets, bedaquiline, and linezolid dosing if significant lactic acidosis develops.
Adverse Reactions
The most common adverse reactions (≥10%) are peripheral neuropathy, acne, anemia, nausea, vomiting, headache, increased transaminases, dyspepsia, decreased appetite, rash, pruritus, abdominal pain, pleuritic pain, increased gamma-glutamyltransferase, lower respiratory tract infection, hyperamylasemia, hemoptysis, back pain, cough, visual impairment, hypoglycemia, abnormal loss of weight, and diarrhea.