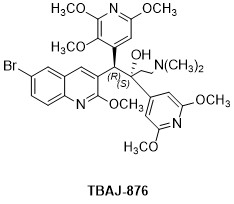
NC-009
Background:
NC-009 is a pan-Phase 2 clinical trial that incorporates elements of Phase 2a, b and c trials. The aim of NC-009 is to evaluate the safety and efficacy of a combination of TBAJ-876, pretomanid and linezolid for its potential to shorten and improve treatment for both drug-sensitive and drug-resistant tuberculosis (TB). NC-009 evaluates this combination, with different doses of TBAJ-876. Another arm of the trial tests the BPaL regimen against drug-sensitive TB. The study aims to enroll 300 participants with drug-sensitive TB at 21 clinical trial sites in five countries: Georgia, Philippines, South Africa, Tanzania, and Uganda. First patient was enrolled on October 24, 2023.
Read the news announcement: