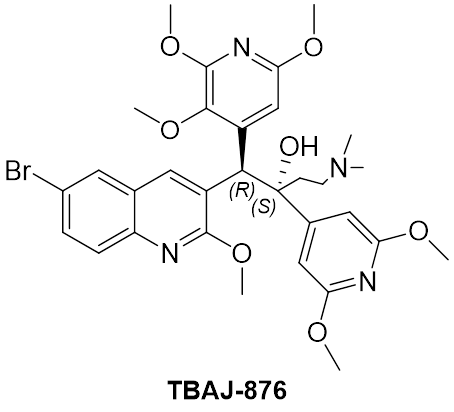
TBAJ-876
A novel anti-tuberculosis (TB) compound, TBAJ-876, has entered a pan-Phase 2 clinical trial (combining components of Phase 2a, 2b, and 2c) which began enrollment in October 2023. Find out more about the NC-009 trial here.
TBAJ-876 is a member of the diarylquinoline class, the same class of drugs to which the anti-TB drug, bedaquiline belongs. Bedaquilne is a component of the WHO-recommended six-month BPaL/M regimens for treating drug resistant TB. Previous data suggests that TBAJ-876 can be more efficacious and potent against TB than bedaquiline, with a lower predicted clinical dose. Similar to TBAJ-587, another next-generation diarylquinoline in development by TB Alliance, TBAJ-876 possesses improved safety properties compared to bedaquiline.