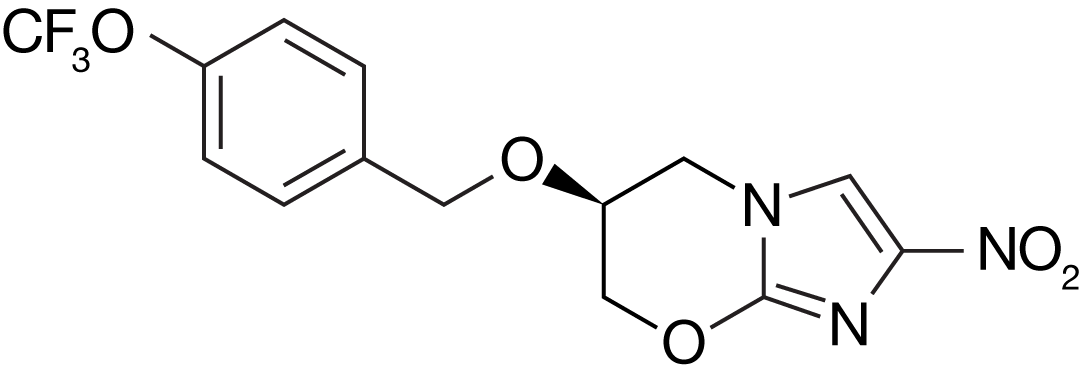
Pretomanid
Names: PA-824
Chemical class: Nitroimidazole
Background
Pretomanid is a nitroimidazole, a class of novel anti-bacterial agents. Pretomanid has been developed by TB Alliance and is approved by the US FDA to treat XDR-TB or treatment-intolerant/non-responsive MDR-TB, in combination with bedaquiline and linezolid, as part of the BPaL regimen. Early in pretomanid's development, it was known as "PA-824."
More information on pretomanid and BPaL can be found here.
Additional Resources:
Peer-reviewed publications
- Treatment of Highly Drug-Resistant Pulmonary Tuberculosis
- Bedaquiline, moxifloxacin, pretomanid, and pyrazinamide during the first 8 weeks of treatment of patients with drug-susceptible or drug-resistant pulmonary tuberculosis: a multicentre, open-label, partially randomised, phase 2b trial
- Efficiency and safety of the combination of moxifloxacin, pretomanid (PA-824), and pyrazinamide during the first 8 weeks of antituberculosis treatment: a phase 2b, open-label, partly randomised trial in patients with drug-susceptible or drug-resistant pulmonary tuberculosis
- Bactericidal Activity of Pyrazinamide and Clofazimine Alone and in Combinations with Pretomanid and Bedaquiline
- 14-day bactericidal activity of PA-824, bedaquiline, pyrazinamide, and moxifloxacin combinations: a randomised trial
- Phase II Dose-Ranging Trial of the Early Bactericidal Activity of PA-824
- Early bactericial activity and pharmacokinetics of PA-824 in smear-positive tuberculosis patients
- A Small-Molecule Nitroimidazopyran Drug Candidate For The Treatment Of Tuberculosis
- Progress In TB Drug Development and What Is Still Needed
- Prospects for Clinical Introduction of Nitroimidazole Antibiotics for the Treatment of Tuberculosis
- Preclinical Testing of the Nitroimidazopyran PA-824 for Activity against Mycobacterium tuberculosis in a Series of In Vitro and In Vivo Models
- Bactericidal Activity of the Nitroimidazopyran PA-824 in a Murine Model of Tuberculosis
- Combination Chemotherapy with the Nitroimidazopyran PA-824 and First-Line Drugs in a Murine Model of Tuberculosis
- Powerful bactericidal and sterilizing activity of a regimen containing PA-824, moxifloxacin, and pyrazinamide in a murine model of tuberculosis
- Synthesis, Reduction Potentials, and Antitubercular Activity of Ring A/B Analogues of the Bioreductive Drug (6S)-2-Nitro-6-{[4-(trifluoromethoxy)benzyl]oxy}-6,7-dihydro-5H-imidazo[2,1-b][1,3]oxazine (PA-824)
- Safety, Tolerability, and Pharmacokinetics of PA-824 in Healthy Subjects
- Assessment of the Effects of the Nitroimidazo-Oxazine PA-824 on Renal Function in Healthy Subjects
- Synthesis and Structure-Activity Studies of Biphenyl Analogues of the Tuberculosis Drug (6S)-2-Nitro-6-{[4-(trifluoromethoxy)benzyl]oxy}-6,7-dihydro-5H-imidazo[2,1-b][1,3]oxazine (PA-824)
Clinical Trials
- A Phase 3 Study Assessing the Safety and Efficacy of Bedaquiline Plus PA-824 Plus Linezolid in Subjects With Drug Resistant Pulmonary Tuberculosis
- Shortening Treatment by Advancing Novel Drugs (STAND)
- Evaluation of 8 Weeks of Treatment With the Combination of Moxifloxacin, PA-824 and Pyrazinamide in Patients With Drug Sensitive and Multi Drug-Resistant Pulmonary Tuberculosis (TB)
- Evaluation of Early Bactericidal Activity in Pulmonary Tuberculosis With(J-M-Pa-Z)
- Evaluation of Early Bactericidal Activity in Pulmonary Tuberculosis With Clofazimine (C)-TMC207 (J)-PA-824 (Pa)-Pyrazinamide (Z) (NC-003)
- Evaluation of Early Bactericidal Activity in Pulmonary Tuberculosis
- PA-824-CL-007: Phase IIa Evaluation of Early Bactericidal Activity in Pulmonary Tuberculosis
- Evaluating the Safety and Drug Interaction of PA-824, an Investigational Tuberculosis Medication, Together With Efavirenz, Ritonavir-Boosted Lopinavir, or Rifampin
- Effect of PA-824 and of PA-824 Plus Moxifloxacin on the QTc Interval in Healthy Volunteers
- Food Effect Study on the Bioavailability and PK of PA-824 Tablets in Healthy Adult Subjects (CL-009)
- Food Effect Study on the Bioavailability and PK of PA-824 Tablets in Healthy Adult Subjects (CL-003)
- Evaluation of the Pharmacokinetic Interaction Between PA-824 and Midazolam
FDA Briefing Materials