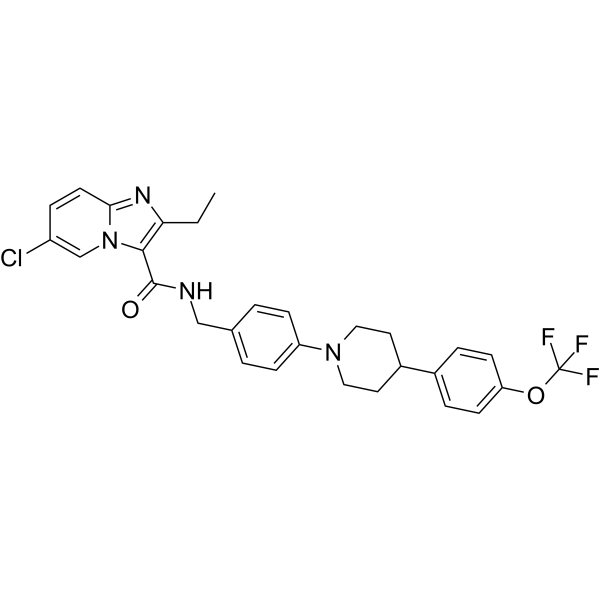
Q203
Names: Telacebec
Chemical class: midazopyridine amide
Background
Telacebec (Q203) is a first-in-class drug candidate against Mycobacterium tuberculosis infection. Telacebec (Q203) targets cytochrome bc1 complex and shows potent activity against multidrug-resistant tuberculosis (MDR-TB).
Telacebec(Q203) also has been found effective against buruli ulcer (Mycobacterium ulcerans), a chronic, necrotizing disease that affects skin and sometimes bone and can lead to permanent deformity and long-term disability.