Developing New Regimens
TB Medicines for All
Without improved, simpler, safer, and affordable cures for tuberculosis—in all its forms—we cannot eradicate the disease.
TB Alliance has the largest portfolio of potential TB treatments in the world. Our pipeline includes several late-stage drug regimens poised to make a transformative impact on the disease. Our product development strategy, whereby we develop drug combinations instead of individual drugs, shows promise to deliver treatments for drug-sensitive and drug-resistant TB, and unify TB therapy under a common, short and simple cure.
For the latest updates on our projects, please visit our Pipeline page or Clinicaltrials.gov. To schedule a briefing, please contact thomas.lynch@tballiance.org. Consult our open access policy for more information on our commitment to information sharing and transparency.
Novel Regimens Poised for Impact
TB Alliance’s ultimate vision is to develop a completely novel drug combination that can treat all forms of TB. The regimens we are currently evaluating in late-stage clinical trials reflect this goal. The more novel drugs in a regimen, the greater proportion of the population that it will be able to cover.
Our Pipeline
TB Alliance manages the largest pipeline of new TB drugs in history.
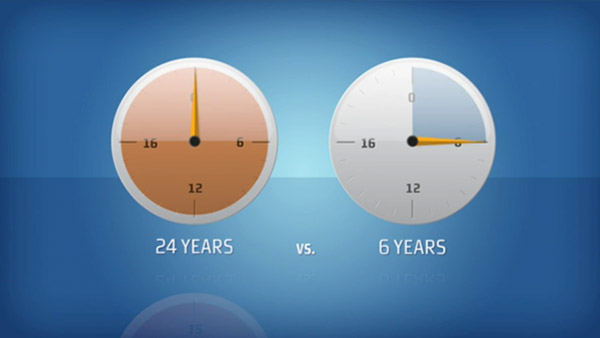
Focus on SimpliciTB:
SimpliciTB is a pivotal clinical trial testing the ability of a novel combination, or regimen, of TB drugs — known as BPaMZ (bedaquiline + pretomanid + moxifloxacin + pyrazinamide) — to shorten the duration of treatment for drug-sensitive (DS-) TB from 6 months to 4 months, and the treatment for drug-resistant TB (including multidrug-resistant TB and mono-resistance to isonaizid or rifampicin) from 9 – 24 months to 6 months. SimpliciTB is targeting enrollment of 450 total participants in at least 26 centers across at least 10 countries in Africa, Asia, Europe and South America.
Advancing the Field
TB Alliance also works to advance the entire field of drug development through the release of several innovative tools, and its leadership role in the Critical Path to TB Drug Regimens (CPTR) Initiative. CPTR is a multi-sectoral initiative that is tackles a wide array of challenges associated with TB drug development, including product development, regulatory science, research resources, and drug sensitivity testing. TB Alliance lead’s CPTR’s “Drug Coalition” arm, which brings drug sponsors work together to assemble and test the most effective treatment regimens.