NC-009
NC-009 is a pan-Phase 2 clinical trial that incorporates elements of Phase 2a, b and c trials. The aim of NC-009 is to evaluate the safety and efficacy of a combination of TBAJ-876, pretomanid and linezolid for its potential to shorten and improve treatment for both drug-sensitive and drug-resistant tuberculosis (TB). NC-009 evaluates this combination, with different doses of TBAJ-876. Another arm of the trial tests the BPaL regimen against drug-sensitive TB. The study aims to enroll 300 participants with drug-sensitive TB at 21 clinical trial sites in five countries: Georgia, Philippines, South Africa, Tanzania, and Uganda. First patient was enrolled on October 24, 2023.
Results of the trial found that sorfequiline had greater activity than bedaquiline, and that the SPaL regimen, a combination of sorfequiline, pretomanid, and linezolid, had greater activity against TB than the standard of care HRZE (isoniazid, rifampin, pyrazinamide, and ethambutol), indicating the potential to shorten treatment time for active TB. In addition, the SPaL regimen had a comparable safety profile to HRZE.
Read the news announcements:
Stage of Development:
Phase 2
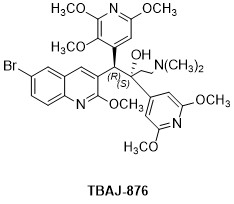
Trial Compounds
This trial has the following compounds:
Trial Regimens
This trial has the following regimens: