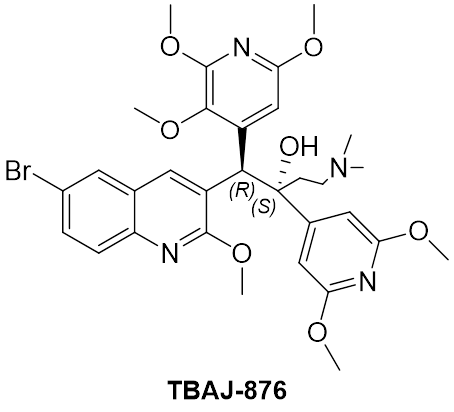
Sorfequiline (TBAJ-876)
Diarylquinoline
A novel anti-tuberculosis (TB) compound, TBAJ-876, has entered a pan-Phase 2 clinical trial (combining components of Phase 2a, 2b, and 2c) which began enrollment in October 2023. Find out more about the NC-009 trial here.
TBAJ-876 is a member of the diarylquinoline class, the same class of drugs to which the anti-TB drug, bedaquiline belongs. Bedaquilne is a component of the WHO-recommended six-month BPaL/M regimens for treating drug resistant TB. Previous data suggests that TBAJ-876 can be more efficacious and potent against TB than bedaquiline, with a lower predicted clinical dose. Similar to TBAJ-587, another next-generation diarylquinoline in development by TB Alliance, TBAJ-876 possesses improved safety properties compared to bedaquiline.
Results of the NC-009 trial further confirmed this potential finding that sorfequiline had greater activity than bedaquiline, and that the SPaL regimen, a combination of sorfequiline, pretomanid, and linezolid, had greater activity against TB than the standard of care HRZE (isoniazid, rifampin, pyrazinamide, and ethambutol), indicating the potential to shorten treatment time for active TB. In addition, the SPaL regimen had a comparable safety profile to the standard of care for people with drug-sensitive TB (DS-TB).
Sorfequiline is expected to enter Phase 3 clinical trials in 2026.
Stage of Development:
Phase 2
Regimen Associations
This compound has appeared in the following regimens
Trial Associations
This compound has appeared in the following trials