TB Alliance Announces New Collaboration with Novartis to Advance the Research and Development of Telacebec for Leprosy
New collaboration aims to advance innovation for a neglected and stigmatizing disease affecting hundreds of…
TB Alliance and Lupin Announce Collaboration to Advance and Commercialize Telacebec for Multiple Neglected Diseases
Partnership will support the development of investigational drug telacebec for the treatment of tuberculosis, leprosy,…
TB Alliance and Asian Development Bank Forge Strategic Partnership to Strengthen Regional Efforts Against Tuberculosis
New York, USA and Manila, Philippines — [Date] — TB Alliance and the Asian Development…
Making Medicines Easier to Take: XTEMP-R Benefits More than Children with TB
For many children, and plenty of adults, swallowing tablets is hard. Ready-made syrups are often…
Promising New Data on Pediatric Use of Pretomanid in Children with Drug-Resistant Tuberculosis Presented at 2025 Union Conference
Positive results mark an important step forward in efforts to develop effective treatments for children…
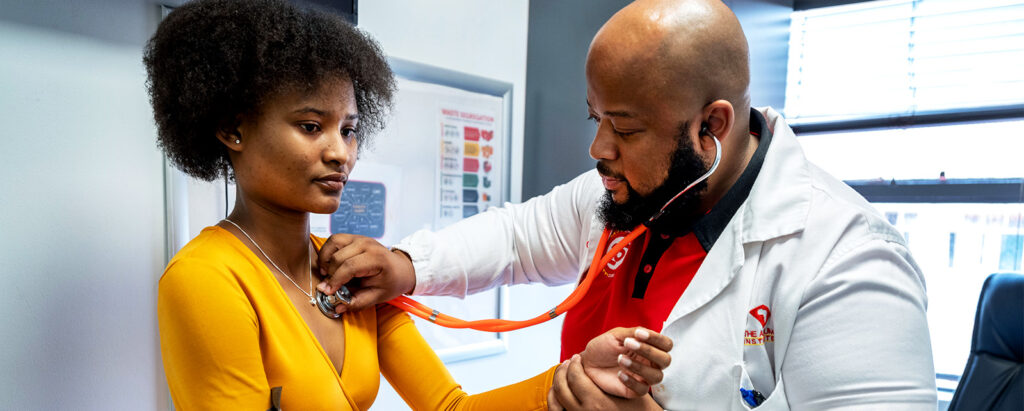
Phase 2 Clinical Trial Results Show Potential to Shorten TB Treatment Time
Thuto Pulane, a TB survivor and participant in the NC-009 trial, at a follow-up appointment…
WHO Report Shows TB Gains, But Funding Gaps Threaten Momentum
TB Alliance is gratified to see clear progress against tuberculosis in the latest WHO Global…
Digital First, Faster Access: How TB Alliance Is Using Tech to Speed BPaL/M to People Who Need It
For decades, breakthrough TB science has too often moved slowly from approval to those in…
TB Alliance Launches Upskill TB Platform in Collaboration with Indonesia’s Ministry of Health and RPRI Foundation
Digital Training Platform, Upskill TB, Strengthens Capacity to Deliver New DR-TB Treatments JAKARTA (October 9,…