
- This event has passed.
Stakeholders Association 2025 Virtual Meeting
February 20, 2025 @ 8:30 am – 10:00 am
TB Alliance at 25: Celebrating Progress, Driving Innovation, and Accelerating TB Eradication
On February 20, 2025, TB Alliance held a virtual meeting of the Stakeholders Association (SHA). The event, “Innovate to Eradicate: The Building Blocks to End TB,” recognized TB Alliance’s 25th anniversary and past contributions to advancing TB research and access, while also setting forth a vision for a new era in TB treatment innovation that could allow the world to think beyond controlling TB and realistically ending TB. The meeting emphasized that achieving this vision requires strong global collaboration, political will, and resources. The speakers highlighted that ultra-short, effective therapies for both active and latent TB are essential pieces in the fight toward eradication and outlined a path toward one-day treatment for latent TB and one-month treatment for active TB, while also accelerating the availability of new TB treatments worldwide.
Mitchell Warren, president of the SHA, opened the meeting by highlighting the SHA’s historic and ongoing role in supporting TB Alliance’s pursuit of its mission—from being a part of TB Alliance’s founding to its impact today. Mr. Warren noted that developments around funding in the TB space and beyond are apt to create additional challenges but stressed the importance of continuing to advance TB innovation and the value of collaboration in doing so.
Dr. Mel Spigelman, president and CEO of TB Alliance, echoed Warren in acknowledging the importance of the SHA and the need for adequate political will and resources to drive the pace of progress in TB and enable those around the world in need of new technologies to rapidly and sustainably access them. Dr. Spigelman recounted TB Alliance’s distinguished record in innovating the TB drug development process, introducing improved therapies for treating pediatric TB and drug-resistant TB, and more recently in leading the fastest and widest roll out a new TB treatment in the modern era.

Dr. Spigelman noted that the previous two and a half decades of leading the global TB drug development space has led to new promise and that with programs currently in development, TB Alliance could soon realize one-month treatment regimens for all active TB (both drug-sensitive and drug-resistant), as well as one-day treatment for latent TB. These technologies, which Dr. Spigelman referred to as the “1 by 1” strategy, are the potential backbone of a toolbox of TB treatments capable of ending TB globally.
With the broader vision established, Dr. Eugene Sun, senior vice president of Research and Development, outlined ongoing research programs and strategies within TB Alliance’s pipeline, and how the organization can deliver the tools of a 1×1 approach. He focused on three key areas: developing a universal regimen, creating a short treatment for latent TB, and exploring delivery methods beyond small molecule therapies—such as long-acting injectables—to dramatically shorten treatment duration.
A universal regimen—a single, novel treatment regimen capable of treating virtually all forms of active TB—is required to simplify TB treatment. Dr. Sun offered an update on the NC-009 trial, which is testing the TBAJ876 + pretomanid + linezolid regimen, noting that enrollment was completed ahead of schedule. While this regimen, as administered in this trial, does not project to be one-month in duration, it does offer the potential to be the first universal regimen. If results from the NC-009 trial are positive, the regimen will continue to the next stage of development.
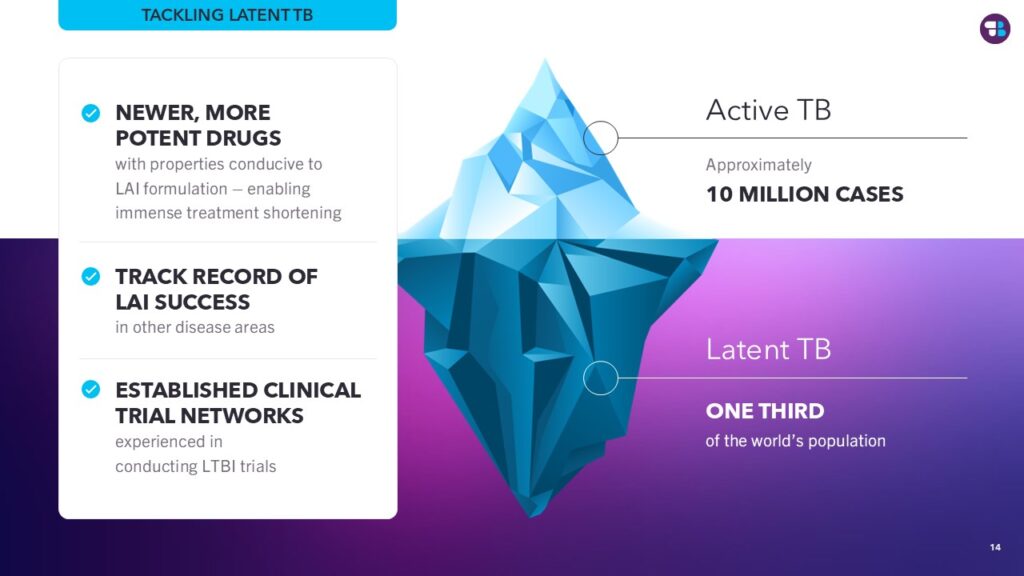
Dr. Sun discussed TB Alliance’s efforts to develop compounds for treating latent TB, a relatively new focus for the organization. The potency of newer drug candidates raises the possibility of a single-day treatment for latent TB. Advancing a new generation of short, simple, and highly effective cures for both latent and active TB is key to tackling the global TB burden from both ends.
Achieving treatment durations as short as one month for active TB and one day for latent TB will likely require formulations beyond traditional pills. One promising innovation is long-acting injectables. Dr. Sun spoke on TB Alliance’s strategies to explore alternative formulations and treatment approaches, highlighting the potential of compounds in its portfolio to support these advancements.
The ultimate goal of any new medical technology is to reach those in need, saving lives and maximizing impact. Sandeep Juneja, senior vice president of Market Access, highlighted TB Alliance’s recent successes in expanding global access to TB treatments and shared key lessons to accelerate future rollouts.
He reviewed the global rollout of pretomanid and the BPaL/M regimens, which became the fastest and most widespread introduction of a new TB treatment in modern history—reducing a typical 7–9-year timeline to just three years. In 2024 alone, 100,000 courses of pretomanid were ordered worldwide.
However, Mr. Juneja emphasized that access can and should be even faster. He outlined strategies to further reduce delays, including earlier engagement with countries, streamlined policymaking, improved procurement and regulatory processes, and better integration of the private sector.
Closing his remarks, Mr. Juneja challenged the community to envision a future where global access to groundbreaking TB treatments—like those in the pipeline—is achieved in just one year, one month, or even one day.
The event concluded with a question-and-answer session covering various topics, including the practical challenges of administering long-acting injectable treatments in low-resource settings, opportunities to align TB Alliance’s vision with the goals of the broader TB research community, as well as the ongoing effects of funding disruptions on both TB research and service delivery.