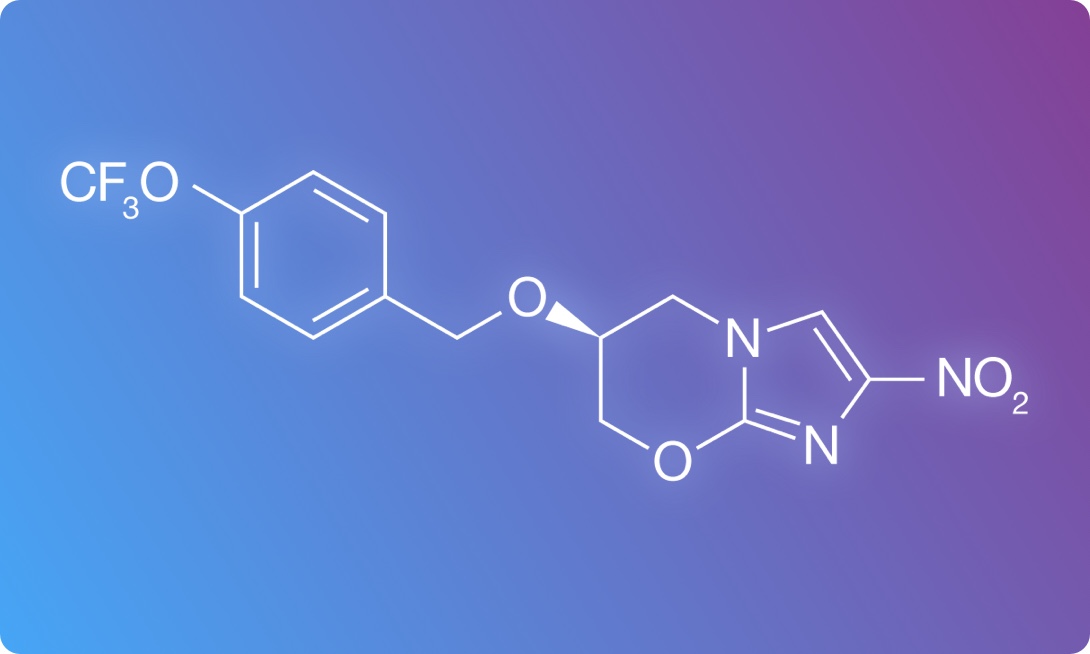
Pretomanid Submitted to FDA
Pretomanid, a novel TB drug developed by TB Alliance, is under regulatory review. In December 2018, TB Alliance submitted its first-ever New Drug Application to the U.S. Food and Drug Administration. Pictured: the chemical structure of pretomanid.

Three Late-Stage Clinical Trials Advancing TB Treatment
TB Alliance is working to introduce promising new TB treatments for all forms of the disease. In 2018, we concurrently led three late stage trials – each expected to offer scientific insights that could significantly impact the global epidemic.
UN High Level Meeting Sparks Global Commitments to Fight TB
In 2018, the United Nations (UN) held a High-Level Meeting (HLM) on Tuberculosis – only the fifth-ever time the UN devoted an HLM to a health issue.


