
“In 2024, we saw the global TB community make real progress—shorter treatments, broader access, and new momentum. But to truly end TB, we need to think bigger and act faster. That’s what our 1×1 strategy is all about: curing latent TB in one day, active TB in one month. It’s bold, it’s necessary, and it’s possible. Together, we can turn this vision into a reality and move closer to a world without TB.”
Mel Spigelman,
President and CEO of TB Alliance
TB remains one of the world’s deadliest infectious diseases – despite being curable.
More than 10 million people fall ill with TB each year, and 1.3 million people die from TB annually. Treatment today is still too long, too complicated, and too out of reach for too many. Our bold 1×1 strategy—treating latent TB in one day and active TB in one month—aims to change that, transforming what’s possible in TB care.

In 2024, we saw real progress: shorter treatments, broader access, collaborative partnerships.
Through initiatives like PeerLINC, LIFT-TB, SLASH-TB, and Fast Track the Cure, we helped countries scale six month DR-TB regimens and expand access to improved cures. We’re also driving forward the next wave of innovation—long-acting therapies, new drug candidates, and pioneering clinical trials—all aligned with the 1×1 vision of radically simpler, shorter, and faster cures for all forms of TB.

Innovation alone won’t end TB; true global commitment is needed.

“What continues to impress me is not just the ambition of TB Alliance, but the discipline and drive behind it. This team is tackling one of the world’s toughest health challenges with clarity, urgency, and a focus on impact. It’s not just about effort – it’s about results.”
David Norton,
Chair, TB Alliance Board of Directors
From Early-Phase Discovery Research to Product Approval: Charting the Future of TB Care

NC:009 Trial of First Potential Universal Regimen Completes Enrollment ahead of Schedule
The NC-009 clinical trial reached full enrollment in August 2024, ahead of schedule. The trial, designed to select the optimal dose of TBAJ-876 in combination with pretomanid and linezolid, also evaluated the treatment shortening potential of this combination. This trial also compares TBAJ-876PaL to BPaL and HRZE, the present standards of care in both the DR and DS populations, respectively, thus offering evidence for the first-ever universal treatment, capable and acceptable for treating both DS-TB and DR-TB. Full enrollment marks a key milestone, bringing the study closer to generating critical data that could support the development of faster, safer, more effective TB regimens and improve outcomes for people with TB worldwide.
Progress in Long-Acting Injectables Unlocks Potential to Dramatically Shorten TB Treatment
Long-acting injectables (LAIs), which are designed to maintain therapeutic drug levels for months with a single injection are emerging as a promising strategy in TB treatment research. TB Alliance has begun advancing LAI formulations that could markedly simplify treatment and improve adherence. These innovations could be transformative for TB control, especially in hard-to-reach populations, by reducing the burden of daily pills and enhancing treatment completion rates. By combining new drugs and regimens in our pipeline with innovative technologies like LAI, we believe it is possible to achieve durations of treatment administration to as short as one month for active TB and one day for latent TB.

TBAJ-587 Completes Phase 1 Study through ERA4TB
The ERA4TB consortium has successfully completed the Phase 1 study for TBAJ-587, a promising new TB drug candidate and another second-generation diarylquinoline. This achievement advances TBAJ-587 into later-stage clinical development, where it will be evaluated for its potential to contribute to shorter, simpler, and more effective TB treatments, and reflects recent growth in the global pipeline of new TB drugs.
Beyond TB: TB Alliance’s Launches its First Trial for Buruli Ulcer
TB Alliance has commenced a new clinical trial evaluating Q203 (telacebec), a novel compound, for the treatment of Buruli ulcer, a devastating neglected tropical disease that can cause severe skin and soft tissue damage. Conducted in collaboration with the Borstel Research Center and partners in Australia, the study marks the first time Q203 has been tested in people with Buruli ulcer. Q203’s promising profile may offer a simpler, more effective, shorter course of treatment compared to current therapies, reducing treatment time by more than half and significantly improving outcomes and quality of life. TB Alliance also continues to develop Q203 for tuberculosis, as well as leprosy, forging a co-development strategy that leverages synergies and maximizes possible global health impact.
Phase 1 Results for TBAJ-876 Published, Confirming Favorable Safety Profile

SPOTLIGHT: Professor Harriet Mayanja-Kizza
Professor Harriet Mayanja-Kizza, based at the Uganda Case Western Reserve University Research Collaboration program in Uganda, is a primary investigator for the NC-009 clinical trial. A long-standing champion of TB research, she is dedicated to finding faster, safer cures for people with TB worldwide.

“Even as TB regimens improve, we must continue investing in research for new treatment options. Trials like NC-009 are critical to finding solutions that can reduce treatment time even further and ultimately help us eliminate TB.”
Prof. Harriet Mayanja-Kizza
Primary Investigator, NC-009 Clinical Trial
A New Era for DR-TB: Rapid Rollouts, Rising Success Rates, and Global Leadership

PeerLINC Advances DR-TB Treatment with Rapid and Cost-Effective Technical Assistance
In its first year of operations, the PeerLINC Knowledge Hub, a collaboration between TB Alliance and the Tropical Disease Foundation, in close coordination with the Department of Health, Philippines has supported nine countries in rapidly adopting or scaling up shorter, more effective six-month BPaL/M treatment regimens for DR-TB. PeerLINC’s model has been able to provide fast, cost-effective, peer-led technical assistance to participating countries. Further, PeerLINC has removed barriers to accessing this type of support that has traditionally disadvantaged smaller countries with lower disease burdens. All countries that have participated in PeerLINC are in the process of scaling up six-month DR-TB treatments. PeerLINC is funded by the Australian government’s Department of Foreign Affairs and Trade.

180,000
patients
100,000 Treatments Across 100 Countries: Scaling Up BPaL/M for Global DR-TB Impact
The global rollout of pretomanid and BPaL/M is the fastest TB drug introduction in modern history. In 2024 alone, the second year since WHO guidelines were issued for programmatic use, more than 100,000 courses were ordered across approximately 100 countries—covering 60%+ of DR-TB cases treated annually. TB Alliance launched a series of initiatives like LIFT-TB, PeerLINC, Fast Track the Cure, and SLASH-TB, which, supported by our commercialization and a diverse base of local implementation partners, and a focus on affordability have helped expand access rapidly. While TB Alliance remains committed to further expanding access so all who can benefit from these treatments receive them, we are encouraged and heartened by the real-world impact pretomanid and BPaL-based regimens are already having on the health of so many people and health systems around the world at an unprecedented pace for novel TB therapies.
LIFT-TB Countries Honored for Leadership in Expanding DR-TB Treatment
The LIFT-TB initiative played a key role in expanding global access to six-month DR-TB regimens and spearheading the fastest and widest rollout of a new TB drug in modern history. Operations research conducted through LIFT-TB generated experience and field evidence—a 90+% treatment success rate—helping pave the way for BPaL/M use in seven high-burden countries and creating real world examples for other countries to follow. LIFT-TB alumni are now global leaders in DR-TB treatment and key contributors to PeerLINC, helping other nations implement new regimens. At the 2024 Union Conference, TB Alliance honored LIFT-TB alumni for their outstanding leadership in advancing TB care.






SPOTLIGHT: Peru Implements new DR-TB treatments Resulting in Improved Treatment Outcomes and Experiences
Shortly after PeerLINC’s formal launch, it worked with Peru to help local officials implement new DR-TB treatments in Peru. This assistance has been coupled with local-led initiatives and additional support from TB Alliance’s Market Access team. More than 1,200 Peruvians had benefited from six-month DR-TB regimens within the first year, and treatment success rates have risen from 60% to 90%. Successes like Peru’s dramatic rise in treatment outcomes highlight the real-world impact.

“The implementation of BPaLM is making a tremendous difference in the experiences of people being treated for drug-resistant TB and those providing that treatment. Participating in PeerLINC has helped accelerate the pace at which we’ve been able to scale these new regimens.”
Dr. Valentina Alarcón
Executive Director of Tuberculosis Prevention and Control, Ministry of Health, Peru
Global Community is the Key to Ending TB
Innovation alone won’t end TB. Achieving meaningful progress requires global solidarity—and that means coordinated action across every sector. From researchers developing new treatments to clinicians implementing them in the field, from policymakers shaping health priorities to advocates raising awareness, and most importantly, the voices of people directly impacted by TB, every part of the TB community has a crucial role. Strengthened partnerships and collaborations are essential to ensure that scientific breakthroughs lead to tangible, life-saving changes. Only through sustained, united efforts can we reduce TB to a manageable threat and work together toward its elimination.











TB Alliance Unveils 1×1 Strategy to Accelerate TB Innovation
TB Must Be Central to the Fight Against AMR
Drug-resistant TB accounts for about
1 in 3
deaths from antimicrobial resistance
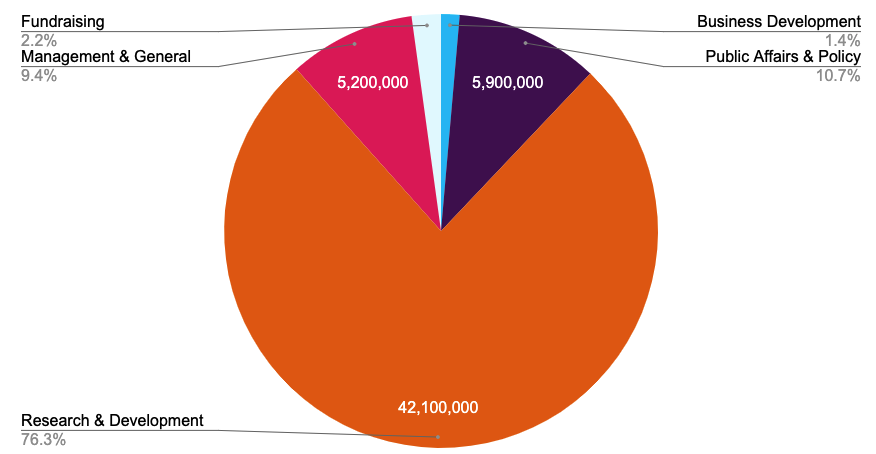
Our Financials
Despite the growing impact of new TB treatments and scientific promise of next generation treatments, global TB research funding continues to decline. The 2024 G-FINDER report showed that the world did not rebound from the previous year’s 10% decrease in neglected disease R&D. Funding for product development partnerships (PDPs) also continues to fall, despite being proven engines of prolific and cost-effective global health innovation. Investment in work like that of TB Alliance remains one of the world’s best investments; a recent Impact Global Health report measured the ROI of neglected disease R&D at greater than 400-to-1. While financial challenges restrain and interrupt the global TB response, unnecessarily increasing risks to global health, prosperity, and security, we remain convinced ending TB is possible and committed to advancing TB treatment and ensuring access to all who need it.
SPOTLIGHT: 15,000 Strong, Fast Track the Cure Showcased at the UNHLM on AMR
The Fast Track the Cure movement is a community led initiative that brings groups around the world together to educate various stakeholders about six-month DR-TB treatments and advocate for access to these treatments for all. Amid the United Nations High Level Meeting on Antimicrobial Resistance, Fast Track the Cure presented a petition, signed by more than 15,000 people across more than 150 countries, urging global leaders to commit to providing access to six-month drug-resistant TB treatments for all by the end of 2024. The attendees, including heads of state, funding organizations, thought leaders, activists, and community representatives erupted into a rousing chant of, “Yes we can – End TB!”
