Patient Stories & Partner Quotes
Additional Resources
About TB Alliance
TB Alliance is a not-for-profit organization dedicated to finding faster-acting and affordable drug regimens to fight TB. Through innovative science and with partners around the globe, we aim to ensure equitable access to faster, better TB cures that will advance global health and prosperity. TB Alliance operates with support from Australia’s Department of Foreign Affairs and Trade, Bill & Melinda Gates Foundation, Cystic Fibrosis Foundation, European & Developing Countries Clinical Trials Partnership, Germany’s Federal Ministry of Education and Research through KfW, Global Health Innovative Technology Fund, Indonesia Health Fund, Irish Aid, Medical Research Council (United Kingdom), National Institute of Allergy and Infectious Disease, Netherlands Ministry of Foreign Affairs, United Kingdom Department for International Development, and the United States Agency for International Development.
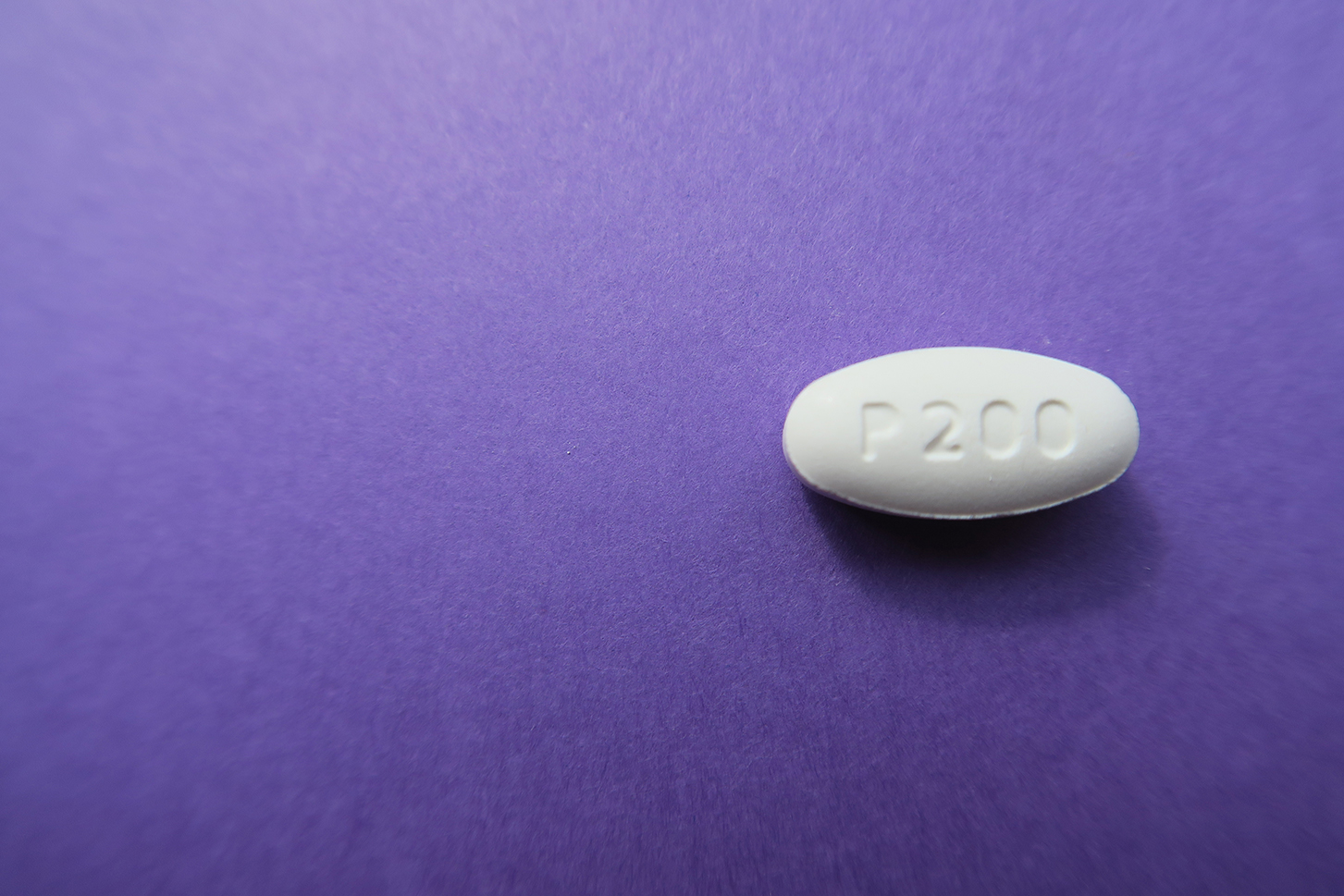
Download a photo of pretomanid here:
Mel Spigelman
President and CEO
Dr. Spigelman is the President and Chief Executive Officer of TB Alliance, and a Member of its Board of Directors. Prior to being appointed President and CEO in 2009, Dr. Spigelman served for five and a half years as the Director of Research & Development at TB Alliance. He was instrumental in forging key organizational partnerships and building the pipeline of TB drug candidates. Notably, Dr. Spigelman was a leader in developing a regimen-based paradigm of TB drug development – a faster and more efficient approach, which is emerging as the gold standard within the TB drug research field.
Dr. Spigelman spent a decade managing drug R&D at Knoll Pharmaceuticals (a division of BASF Pharma) prior to joining TB Alliance. As Vice President of R&D at Knoll for eight years, Dr. Spigelman supervised all R&D activities from basic discovery to regulatory approval and Medical Affairs. He established global R&D processes as part of Knoll’s senior R&D management team, oversaw a marked increase in US regulatory filings and approvals, and supervised joint R&D programs with multiple other pharmaceutical companies.
Dr. Spigelman received his undergraduate degree from Brown University and his medical degree from the Mt. Sinai School of Medicine where he specialized in Internal Medicine, Neoplastic Diseases and Preventive Medicine. Dr. Spigelman holds board certifications from the American Board of Internal Medicine, the American Board’s Subspecialty Board of Medical Oncology, and the American Board of Preventive Medicine and was the recipient of the American Cancer Society Clinical Oncology Career Development Award (1985-1988).
Presently, Dr. Spigelman is Co-Chair of the Working Group on New Drugs of the WHO Stop TB Partnership, and is a member of the Governing Board of the Tres Cantos Open Lab, GlaxoSmithKline.
Francesca Conradie
Principal Investigator, Nix-TB Clinical Trial
Dr. Conradie is the principal investigator of Nix-TB, TB Alliance’s first Phase 3 trial evaluating the BPaL regimen. Throughout her career, Dr. Conradie has been a part of the research agenda that brought effective antiretroviral therapy to millions of South Africans living with HIV. Now, she is focused on TB research, especially new treatment strategies for drug-resistant TB.
Dr. Conradie obtained her MBBCh at the University of the Witwatersrand, Johannesburg, South Africa.
Dan Everitt
Vice President and Senior Medical Officer
Dr. Everitt serves as Senior Medical Officer and works with the clinical development of products in the TB Alliance portfolio. Prior to joining TB Alliance, he spent 10 years in Johnson & Johnson’s pharmaceutical sector. There, Dr. Everitt served in various roles, including as Vice President and Global Head of Clinical Pharmacology and Experimental Medicine at Johnson & Johnson Pharmaceutical Research and Development, and as Vice President of Safety Governance. Before working at Johnson & Johnson, Dr. Everitt spent 10 years in clinical research and development at SmithKline Beecham Pharmaceuticals. For six years, he was an Investigator in the Clinical Pharmacology Research Unit based at the University of Pennsylvania, and subsequently led Phase 2-4 development projects based in Harlow, United Kingdom, in the areas of neurosciences and anti-infectives.
Dr. Everitt gained his undergraduate degree from Duke University and his medical degree from Harvard Medical School. After an Internal Medicine residency at the Massachusetts General Hospital, he was a Fellow at Harvard in Geriatric Medicine and Clinical Epidemiology. Dr. Everitt is Board Certified in Internal Medicine, Geriatric Medicine and Clinical Pharmacology. He served on the faculty of departments of medicine at Harvard Medical School and at the University of Pennsylvania. In 2011, Dr. Everitt completed training and received a Diploma in Tropical Medicine and Hygiene from the London School of Hygiene and Tropical Medicine. Just prior to joining the TB Alliance, Dr. Everitt spent several months as a volunteer physician working in mission hospitals in Kenya. He is an author or co-author of 50 publications in peer-reviewed journals in the fields of Clinical Pharmacology and Pharmacoepidemiology.