TB Alliance is grateful to all partners and stakeholders who have helped and supported us to get to this stage, especially our clinical trial participants. This important milestone demonstrates how our not-for-profit product development partnership (PDP) model works – as we move closer to our goal to eliminate TB.
TB Alliance is dedicated to the discovery, development and delivery of better, faster-acting and affordable TB drugs that are available to those who need them.
Dr. Francesca Conradie, principal investigator of Nix-TB

“Until very recently, people infected with highly drug-resistant TB had poor treatment options and a poor prognosis. This new regimen provides hope with 9 out of 10 patients being cured with this short, all-oral regimen.”
Nick Herbert, MP of the UK and co-chair of Global TB Caucus
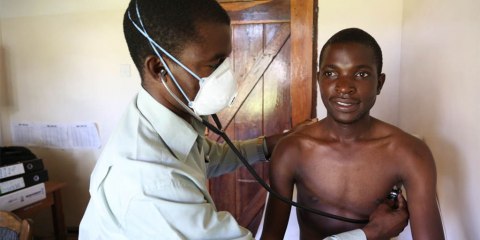
“Tuberculosis has been neglected for decades, and until recently, it had reached the point where extensively drug-resistant TB (XDR-TB) became synonymous with either a death sentence, or years of painful, often intolerable treatment.
The threat of TB drug resistance can only be tackled through a concerted global effort with ambitious and concrete actions from the international community. This includes investing heavily in the development of new drugs, vaccines and diagnostics.
The FDA’s approval of pretomanid is a direct result of the type of coordinated effort we need to fight TB. The UK government, together with other countries and stakeholders, has invested in the development of this drug as part of a new regimen and it represents a major step forward in the fight to end TB.”
Sigrid Kaag, minister for Foreign Trade and Development Cooperation, Netherlands

“I am very proud that we have contributed to this important innovation. As tuberculosis is still a disease of developing countries it is good to see that public private partnerships work and lead to very relevant interventions “ said minister Kaag (Minister for Foreign Trade and Development Cooperation). “This treatment is a new instrument in the fight against Tuberculosis and antimicrobial resistance. We cannot reach SDG3 without innovation.”
Lelio Marmora, executive director of Unitaid

“With the BPaL regimen, the world now has an additional treatment for highly drug-resistant forms of TB – a key breakthrough to fight the most deadly infectious disease. We welcome the development of more options to treat TB as a means of jointly advancing global elimination goals.”
Doris Rouse, PhD, vice president at RTI International

“FDA approval of this breakthrough new treatment for XDR-TB demonstrates the important role that TB Alliance and other public-private partnerships have in developing solutions for critical health needs. TB Alliance’s impressive success in bringing this new drug to market provides an effective tool in fighting the growing, worldwide threat of drug-resistant TB. Our team at RTI International, a non-profit independent research institute, is very proud to have collaborated closely with TB Alliance on the development of pretomanid for almost 20 years. RTI assisted TB Alliance in early due diligence, planning and managing the preclinical studies and has served as a regulatory contact for TB Alliance since the pretomanid IND was submitted to FDA in 2005.”
Trevor Mundel, president, Global Health at the Bill & Melinda Gates Foundation

“Many patients suffering from XDR-TB have limited treatment options. The BPaL regimen could have a potentially transformative impact on their lives. It’s solutions like these, and the investments and partnerships behind their development, that are urgently needed to address all forms of TB.”
Alok Sharma, international development secretary at DFID

“I am proud that our long-term support has contributed to the development of this ground-breaking treatment. The UK is already leading the way to tackle TB through investment in research and development, and our scientists continue to develop new TB drugs to treat the millions of people still suffering with the disease.”
Robin Davies, head of the Indo-Pacific Centre for Health Security at DFAT

“With nearly half of the world’s people with drug-resistant TB living in the Indo-Pacific region, ending the TB epidemic is a priority for Australia. Our experience working with countries like Papua New Guinea has shown us how important it is to find ways to make TB treatment easier, faster, and more effective. This is precisely what pretomanid and the BPaL regime promise to do. We welcome the FDA approval, and look forward to supporting the TB Alliance and partner countries in our region to make the BPaL regime available to patients as soon as possible.”
Bernard Pécoul, executive director of the Drugs for Neglected Diseases initiative (DNDi)

“This new drug from TB Alliance ushers in a long-due transformation of XDR-TB treatment. The new regimen should be incorporated into the TB programs without delay.”
Paul Sommerfeld, chair of TB Alert

“We in TB Alert, the United Kingdom’s tuberculosis not-for-profit active in Africa, India, Eastern Europe, and Britain itself, are greatly encouraged that pretomanid has now received FDA approval. We have long appreciated the efforts of the TB Alliance in developing new medications to treat TB.
Extensively drug-resistant (XDR) TB has for too long been a death sentence for far too many people. With pretomanid and other new drugs, cure rates for XDR-TB can be greatly increased. If there is also political will to introduce these drugs in every country, local communities and the individuals within them facing treatment can truly believe that they will be cured and in much shorter time with safer, more tolerable medication than those used in current regimens.”
Paul Stoffels, M.D., vice chair of the Executive Committee and Chief Scientific Officer, Johnson & Johnson

“Today’s approval of a novel treatment regimen for highly drug-resistant forms of TB gives new hope to patients. Continued investment from all sectors will accelerate the development and delivery of urgently needed tools for TB. At Johnson & Johnson, we are committed to doing our part to make TB history.”
Zeda Rosenberg, ScD, CEO of the International Partnership for Microbicides

“This important new TB treatment option is a testament to the power of public-private partnerships to tackle the world’s most pressing health challenges. TB Alliance and its partners brought together the science, financing and hard work necessary to develop an urgently needed product that will improve patients’ lives and boost the global fight against TB.”
Kitty van Weezenbeek, executive director KNCV Tuberculosis Foundatio

“KNCV congratulates the TB Alliance and all others involved with this game changing innovation in the treatment of highly drug-resistant TB patients. The fully oral and relatively short BPaL regimen developed by the TB Alliance will not only bring hope and cure to patients, but also facilitate delivery of care by individual health workers and an effective M/XDR-TB response of health systems. KNCV has introduced and scaled up new drugs and regimens in many countries and acknowledges the breakthrough that this much shorter and injectable free regimen will bring. The FDA endorsement of the BPaL regimen marks the beginning of a new era in which we expect further developments in the coming years. KNCV stands ready to support countries to introduce and quickly scale up this life saving treatment for all eligible patients, generate more evidence, while ensuring the responsible use of Pretomanid based regimens. After all, we need to protect our new drugs! We look forward working with all stakeholders on BPaL. No time to waste.”
Jamie Bay Nishi, director of Global Health Technologies Coalition

“We welcome this approval as it shows the real-world impact of US government investment in finding new cures and vaccines for the world’s deadliest diseases. Amazingly, it’s the first time that a treatment for XDR-TB infections has been recognized for actually working—no other treatment had demonstrated any consistent effectiveness.”
José Luis Castro, executive director of the International Union Against TB and Lung Disease
“The Union welcomes a new shorter all oral regimen for XDR-TB. This represents a step change in the treatment of this severe form of TB removing injectable agents and shortening the duration from two years to six months. Shorter, more effective regimens such as these are better for patients as well as being easier for National TB Programmes to adopt and scale up to offer effective treatment options to all patients. The development of pretomanid as part of a new regimen for one of the hardest to treat forms of TB shows the importance of investing in TB research and development. This investment not only needs to continued but increased to close the $1.3 billion funding gap as highlighted in TAG’s R&D report. We hope that with continued increased investment and the registration of pretomanid as part of the BPaL is the first of many new regimens improving the outcomes for people with TB.”
Dr. Sanjay Sarin, head of FIND India

“The simple BPaL regimen is a tremendous step forward that can save patients with extensively drug-resistant (XDR) TB from a long and complicated treatment journey and greatly improve their chances of survival. To enable the effective and timely implementation of XDR-TB treatment, we are working on several specific diagnostics, including a second-line line-probe assay that has already been rapidly adopted and scaled up in India, and an Xpert XDR-TB cartridge that can be used closer to the point-of-care and is shortly going into multi-country trials. We sincerely congratulate TB Alliance on this achievement and are pleased to continue to work together to deliver these life-saving tools to those in need.”
Mitchell Warren, executive director of AVAC
“Pretomanid and its use in the new BPaL regimen is an important step forward in TB treatment and on the overall global efforts toward TB elimination. Regulatory approval is a huge milestone, but just the beginning of this next, most important part of the process of translating exciting R&D into actual public health impact at the community and individual level. There is much more work to do, but this approval allows the TB Alliance and all of its various stakeholders and partners to move forward to these next steps with urgency.”
Prof Madhukar Pai, MD, PhD, FCAHS
“For too long, patients with drug-resistant TB have had to endure horrible, painful, long therapies. I am thrilled that we now have new TB drugs (e.g. bedaquiline and pretomanid) and regimens that can change this reality. A six-month, all-oral treatment for XDR-TB would be an incredible advance over the current situation, and I hope patients won’t need to wait for too long to access such innovations.”
Stephen Gillespie, Sir James Black Professor of Medicine at the University of St. Andrews

“The continuing high rates of tuberculosis and the apparently inexorable rise in resistant tuberculosis is a challenge to global public health. I am delighted, therefore, to hear of the approval for pretomanid as we are desperately short of new treatments for tuberculosis. This will be a major step forward for treatment shortening and an effective regimen for both XDR-TB and intolerant MDR-TB. The news will give the community hope that we can unlock better, shorter treatment and will provide physicians with more treatment options for patients.”
Dr. Li Liang, chair of the China Medical Association TB Society (CMA-TB) and deputy director of the Beijing Chest Hospital

“The approval of pretomanid as well as its accompanying BPaL regimen (Bedaquline, Pretomanid, Linezolid) marks another milestone in our struggle against the peril of tuberculosis especially MDR/XDR-TB. Pretomanid and BPaL will shorten the current 18-24 month treatment course for MDR/XDR-TB to 6-9 months with its patients taking only 3 oral drugs instead of currently recommended at least 5 agents including injectable, and with higher treatment success. China still ranks high among countries with a high DR-TB burden, the potential introduction of pretomanid and the BPaL regimen could greatly benefit the local patient population especially for MDR/XDR-TB patients who are either intolerant of the currently recommended regimens or simply having no effective drugs available to form a presumably effective regimen.”
Irma Khonelidze, NTP manager, Georgia
“BPaL made it possible to see the END TB perspective in 10 year horizon as defined under the 3.3 SDG, bringing cure rate of the most severe DR TB up to unprecedented 90%. It provides hope for patients in EECA region, which has become MDR TB hot spot and home to the fastest-growing HIV epidemic. Now it’s time to act with a sense of urgency for everybody from the global to the local actors.”
Karl Hofmann, president of Population Services International
“The lives of people suffering from highly drug-resistant TB will get a shot of hope thanks to the FDA’s exciting action on pretomanid. New therapies for the toughest TB cases will not only save lives but also will reduce the burden on entire communities. TB is a disease of poverty, of stigma, the world’s deadliest infectious disease but one that is curable with the right tools and community support. It’s hugely significant that a new therapeutic regimen finally stands ready to make a difference in the lives of people dealing with this ancient killer.”
David Lewinsohn, MD, PhD, chair of the International Health Committee at the ATS and TB researcher

“While TB can be cured using antibiotics, many of these drugs were developed more than 50 years ago. Drug resistance is associated with poor patient outcomes. It is estimated that as many as 20 percent of TB cases are caused by a strain that is resistant to one of the first or second line of TB drugs, and 5 percent are resistant to both INH and Rifampin, a hallmark of multidrug-resistant TB (MDR). Of even greater concern, roughly 10 percent of these are considered extremely drug-resistant (XDR), with additional resistance to second-line drugs. The ATS welcomes the FDA’s approval of pretomanid, TB Alliance’s new treatment for XDR and some forms of MDR-TB. Availability of this novel treatment is a significant advancement in the fight against TB. The ATS congratulates TB Alliance and its partners.”
Vela Mhlola, community engagement officer at TASK Applied Science

“I am living in a country with a high burden of TB. We see friends and family members die because of TB. In communities, TB is seen as discriminating because poor people are mostly the victims of this disease. TB is one of the oldest diseases, new tools and innovations are very important to end this pandemic.
XDR is one of the deadly TB strains – it’s almost a death sentence. People with XDR are staying in isolation, they take treatment for years with no hope to be cured and drinking lots of tablets daily and often even receive an injection. Some of them they experience difficult side effects, like loss of hearing which becomes a disability for them.
I am Community Engagement Officer at TASK Applied Science, TB Alliance gave us an opportunity to participate in the Nix-TB study. Through this study we have experienced life changing moments for people who were waiting to die because there were no options. Participants in this study were receiving 3 tablets for 6 months which is even better than MDR treatment. We see faces of people glow, people standing up and walking, completion in their skin, people putting on weight, people going back to their normal lives, hospitals beds getting empty, most off all people getting cured from XDR. Patients could not wait to meet the criteria so that they can participate in this study.
BPaL regimen will save us lots of resources, less long-distance walks to the clinics, and help destigmatize XDR so that patients can get family support.”
Mel Spigelman, MD, president and CEO of TB Alliance

“The FDA approval represents a victory for the people suffering from these highly drug-resistant forms for the world’s deadliest disease. The associated novel regimen will hopefully provide a shorter, more easily manageable and highly efficacious treatment for those in need.”
