ZeNix
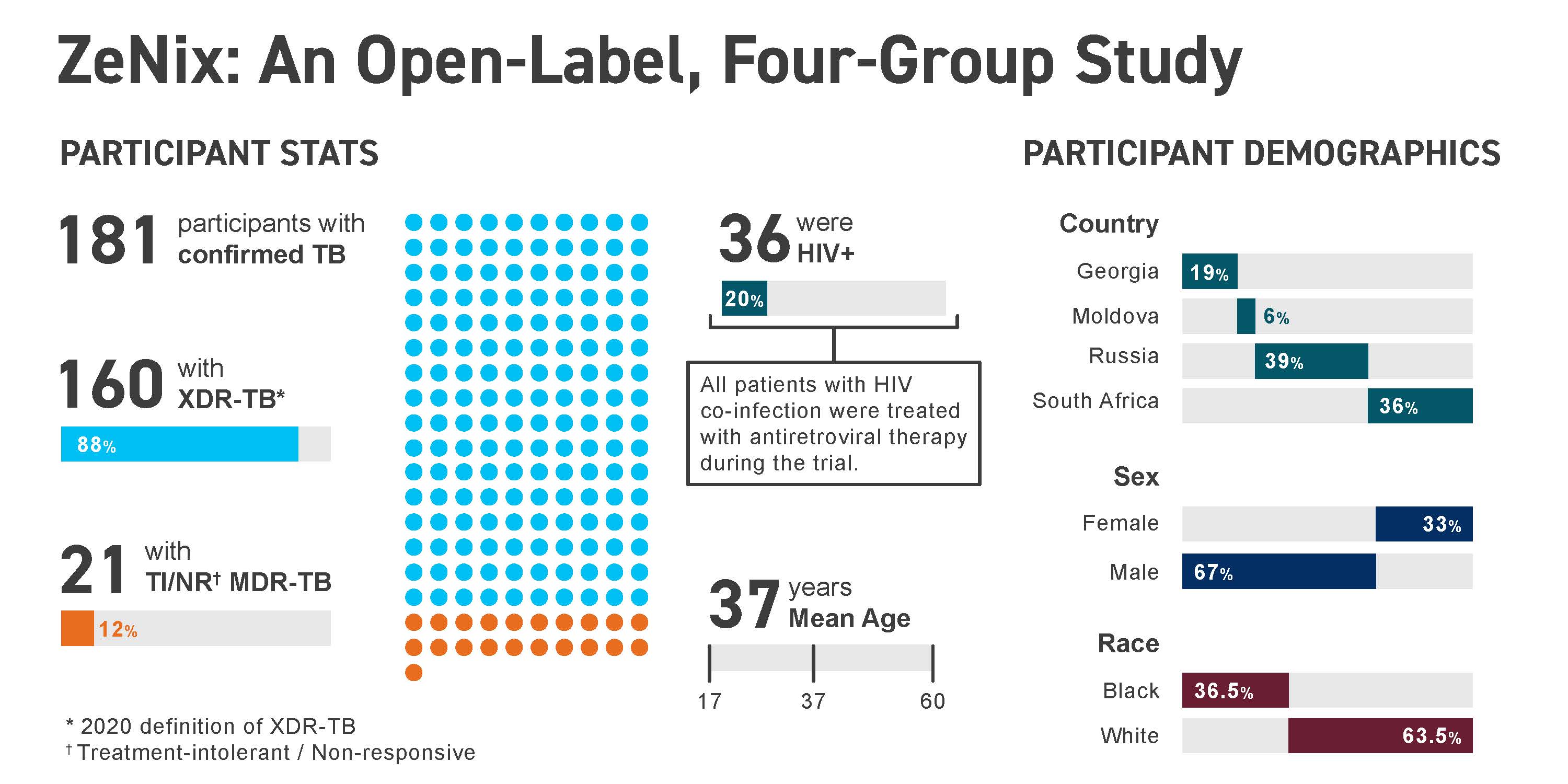
Overview ZeNix is a successor to Nix-TB, a pivotal trial of the BPaL regimen, which was approved by the U.S. FDA in 2019 for the treatment of adults with highly drug-resistant pulmonary tuberculosis (TB). ZeNix tested a version of BPaL with a lower dose and shorter duration of linezolid, to determine whether the efficacy of BPaL can be maintained while reducing toxicity. ZeNix enrolled participants across 11 sites across Georgia, Moldova, Russia, and South Africa.
About the Treatment BPaL consists of bedaquiline, pretomanid, and linezolid. More information about pretomanid and BPaL can be found here.
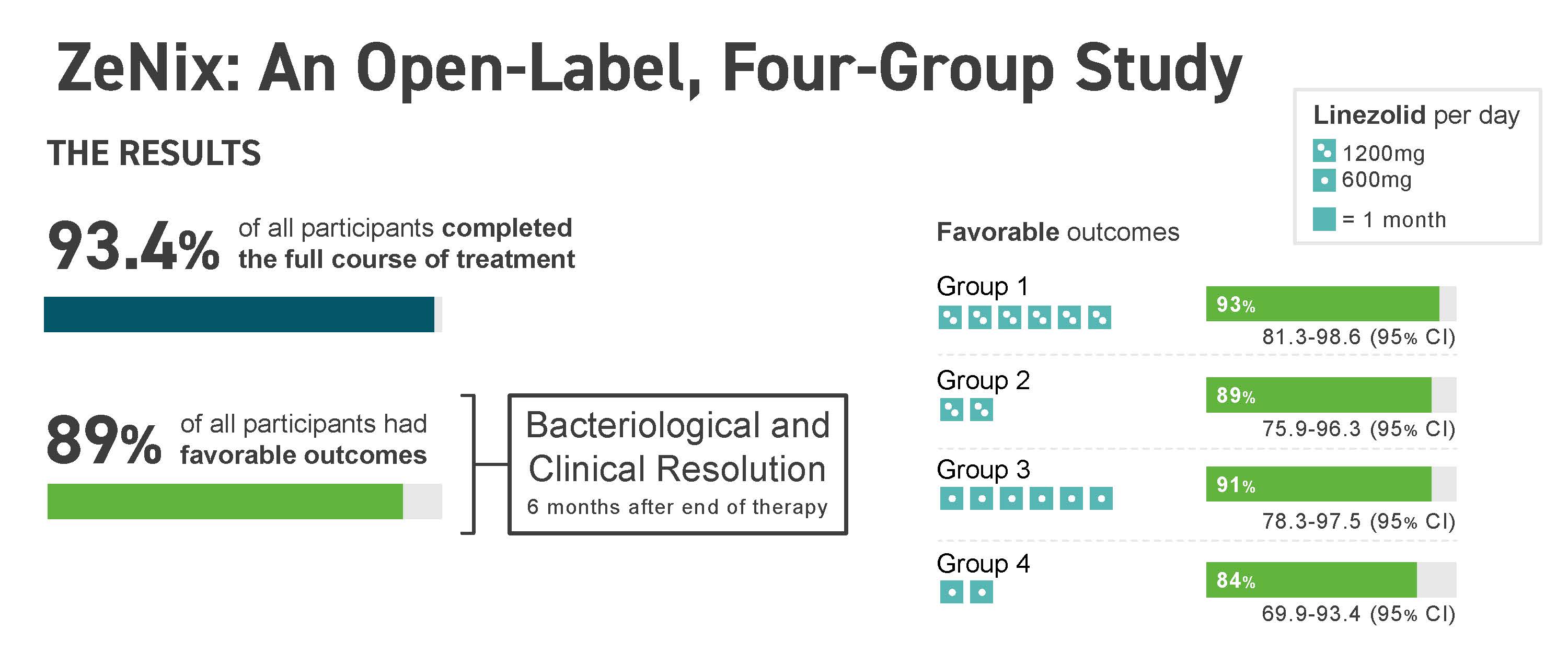
Results Results of the ZeNix trial were published in the September 2022 issue of the New England Journal of Medicine, which reported that the treatment regimen remained effective against highly drug-resistant strains of TB with reduced dosage and/or duration of the linezolid component of the regimen. Along with the maintenance of efficacy, there was a decrease in linezolid-associated side effects that accompanied the reduced dosage or duration of linezolid.
Publications
- Bedaquiline–Pretomanid–Linezolid Regimens for Drug-Resistant Tuberculosis, New England Journal of Medicine, September 2022
- Letter to the Editor: Pretomanid in the Treatment of Patients with Tuberculosis in the United States, New England Journal of Medicine, September 2022
- Editorial: Linezolid for Drug-Resistant Tuberculosis, New England Journal of Medicine, September 2022