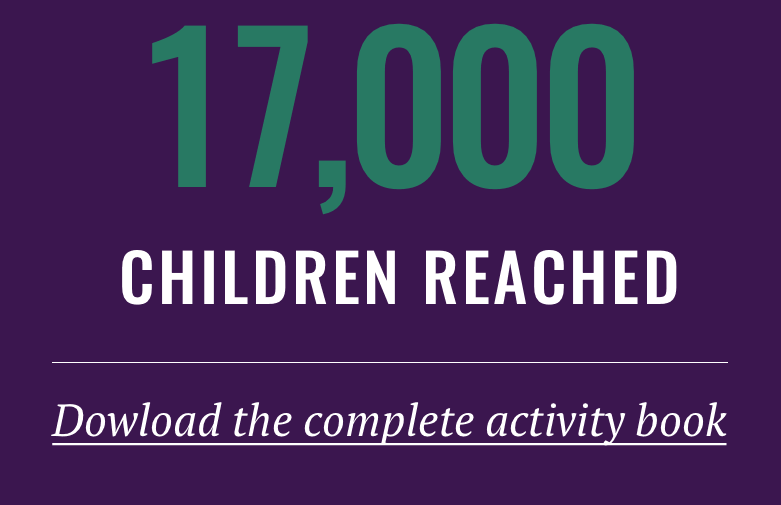Collaborating with Partners
As a product development partnership, TB Alliance works with a wide variety of partners to advance TB drug development in the most efficient way possible.
We depend on the generous support of our donors to develop and introduce the TB regimens that will impact the epidemic. In addition to private organizations like the Bill and Melinda Gates Foundation and the Indonesia Health Fund, national governments play a major role in funding TB Alliance's work.
Over the past year, the government of Germany announced a five year grant in support of developing new TB treatments. Managed by Germany's Federal Ministry of Education and Research (BMBF) and facilitated by the KfW development bank, this investment is part of a new wave of support directed to product development partnerships to fight neglected diseases like TB.
Additional government sponsors of TB Alliance include the Australian Government (DFAT), Japan's Global Health Innovative Technology Fund (GHIT), Irish Aid, the Netherlands Ministry of Foreign Affairs, UK Aid, and three U.S. government institutions: the National Institute of Allergy and Infectious Disease, United States Agency for International Development, and the United States Food and Drug Administration. UNITAID provided major support for our pediatric program.
TB alliance relies on strong private sector partnership, including from pharmaceutical companies, to develop the next generation of TB medicines — especially in the discovery phase. Historically, for every dollar spent, TB Alliance has leveraged an additional $0.68 from in-kind partner support.
TB Alliance is grateful for the ongoing support of our donor organizations, as well as growing support from private donations, and looks forward to leveraging these investments to further advance the TB drug development pipeline.
Partnership with MSF
TB Alliance has provided pretomanid, a drug in the final stages of clinical development, as well as its drug development expertise as a partner on a clinical trial launched by Médecins Sans Frontières/ Doctors Without Borders (MSF).
The study, TB PRACTECAL, a composite Phase II-III trial which will run in three countries, will examine the effectiveness of multiple regimens based on two new classes of tuberculosis (TB) drugs that TB Alliance has been testing in separate trials.
By working alongside partners like MSF on clinical trials, TB is enabling further study of regimens that include promising new TB medicines like pretomanid.
Innovative Collaboration: PepsiCo
In October 2016, TB Alliance and PepsiCo announced a new partnership to explore ways to improve the palatability of TB drugs, which are often bitter-tasting and can be difficult to administer to children.
In the initial stages of this collaboration, TB Alliance asked PepsiCo to evaluate 17 existing TB drugs, which PepsiCo then ran through their proprietary flavor-mapping platform. This work is made freely available to TB Alliance for the purposes of improving drug development and treatment. The results of this study will lead to advice on how to formulate these drugs in a more palatable way. Ultimately, this novel partnership seeks to leverage cutting-edge taste science to eliminate poor palatability — a key obstacle in TB treatment for children and other vulnerable populations.

Dr. Mehmood Khan, Vice Chairman and Chief Scientific Officer, Global Research and Development, for PepsiCo, discusses the importance of its collaboration with TB Alliance and the imperative of improving the palatability of childhood TB medicines.

It's just lovely to think of the fact that children who might otherwise have died or children who might otherwise have gone through months and months of the most painful and often nauseating responses to pills they never wanted to take, that they'll now adjust to a medicine which works.— Stephen Lewis,
co-founder of AIDS-Free World
Engaging Communities
Informed and engaged communities make the development of new TB medicines possible. TB Alliance actively supports a robust community engagement (CE) program, which provides grants to research sites to implement strategies which strengthen relationships with community stakeholders and work to inform and solicit input into clinical trials from communities where the research is taking place. In 2016, over 21 CE programs in six countries were provided grants, guidance and technical assistance to develop site-level CE strategies and aid in the work carried out by Community Advisory Boards. Small grants were also issued to support 30 community events in 9 countries to mark World TB Day with screenings and awareness-raising events.
TB Alliance has been promoting the work in CE throughout the year on its Facebook page. One special highlight included an event that took place in May 2016. TB Alliance partnered with the Western Cape Education Department in South Africa to launch an instructional toolkit for teachers and an activity book for children to foster TB literacy in schools. The materials were provided to 24 schools in South Africa over the course of a two-week roll-out. The roll-out was conducted with partners KickTB&HIV and the South African Tuberculosis Vaccine Initiative (SATVI), which provided soccer-based TB awareness activities and educational materials, reaching a total of 17,000 children.

Indonesia Health Fund Supports Capacity Building and Research
TB Alliance has received funding from the Indonesia Health Fund to coordinate efforts to build capacity to advance TB research in Indonesia. This includes developing clinical trial sites to conduct TB drug studies, as well advancing early research. With Indonesia being one of the highest TB burden countries globally, this is an exciting opportunity to work with partners in the country to improve TB treatment. TB Alliance is also involved in efforts to improve knowledge about TB disease and research in affected communities in Indonesia.
TB Alliance is indebted to its partners, especially all participants in our clinical trials, for the successes of 2016.