



TB, like many other bacterial diseases treated with antibiotics, is growing increasingly resistant to the available drugs that have been in use for decades. Extensively drug-resistant TB, or XDR-TB, is caused by strains of M. tuberculosis that are resistant to at least four commonly used anti-TB drugs. XDR-TB is often considered a death sentence. The proof is in the shocking statistics: Those with XDR-TB, who are diagnosed and receive care, only have about a 15% chance of survival. That's far worse than say, Ebola, where patients have at least a 50% chance of survival.
The narrative used to be that drug-resistant patients defaulted or didn't take their medication properly, and therefore ended up with an extreme form of the disease. That's not necessarily true today. With an estimated 40,000 people infected with XDR-TB today, spread across 105 countries, many patients contract XDR-TB in the same way as other forms of the disease — through the air.
In May 2015, TB Alliance and partners announced the launch of the world's first clinical trial to study an XDR-TB drug regimen with minimal pre-existing resistance. This regimen is known as BPaL. If successful, the injection-free regimen being tested in the so-called Nix-TB clinical trial could transform XDR-TB treatment by significantly improving cure rates with a relatively short, simple, and effective regimen. By reducing the complexity and dramatically reducing the cost, the regimen being tested in the trial offers the promise to facilitate the global implementation of XDR-TB treatment in resource-poor nations.
Nix-TB tests a three-drug regimen consisting of bedaquiline, which received conditional regulatory approval in several high-TB disease burden countries; the novel antibacterial drug compound pretomanid, which is being tested in multiple clinical trials for TB; and linezolid, an oxazolidinone that has been used off-label to treat TB. The trial brings hope to those who have no other treatment options. The trial includes patients as young as 14.
Nix-TB is an open-label study that aims to cure people with XDR-TB (or those who cannot tolerate the current MDR-TB treatment) in six to nine months. After completing treatment, participants are monitored for two years to ensure they do not relapse.
The trial has an adaptive design; if improved drugs or more effective schedules of drug administration become available during the course of the study, they can be incorporated into the trial. For example, the study has evolved to take into account findings from a dose-ranging study of linezolid.
Nix-TB is a partnership between TB Alliance, the sponsor of the trial; Janssen Pharmaceuticals, the discoverer of bedaquiline; and the initial sites in South Africa where the study is being conducted (Sizwe Hospital and TASK at Brooklyn Chest Hospital). The study will expand to include other partners and sites.
Currently, there are nearly 40 patients enrolled in the trial; 13 patients have completed the regimen and have been able to go home. These patients will now be monitored for potential relapse for two years as part of the trial. Over the course of 2016, we expect to receive results for those patients who have completed their treatment with six month follow up. The data will allow TB Alliance to determine next steps, if warranted, to advance testing of the regimen.
To learn more about this trial from the lead investigator, click here.

A recent report noted that drug-resistant TB is a significant driver of the trillions that antimicrobial resistance is expected to cost the world over the coming decades. That takes the form of global productivity as well as lives lost.
The good news is that, with the further rollout and availability of the GeneXpert diagnostic test, more patients today are being diagnosed with drug-resistant TB. However, there is still no effective medicines for them.
XDR-TB treatment
today is necessarily one of last resort. There is no standard regimen — healthcare providers often use a mixture of
whatever drugs patients are susceptible to.
This could look similar to MDR-TB treatment, however, the regimens are typically more toxic and less efficatious.
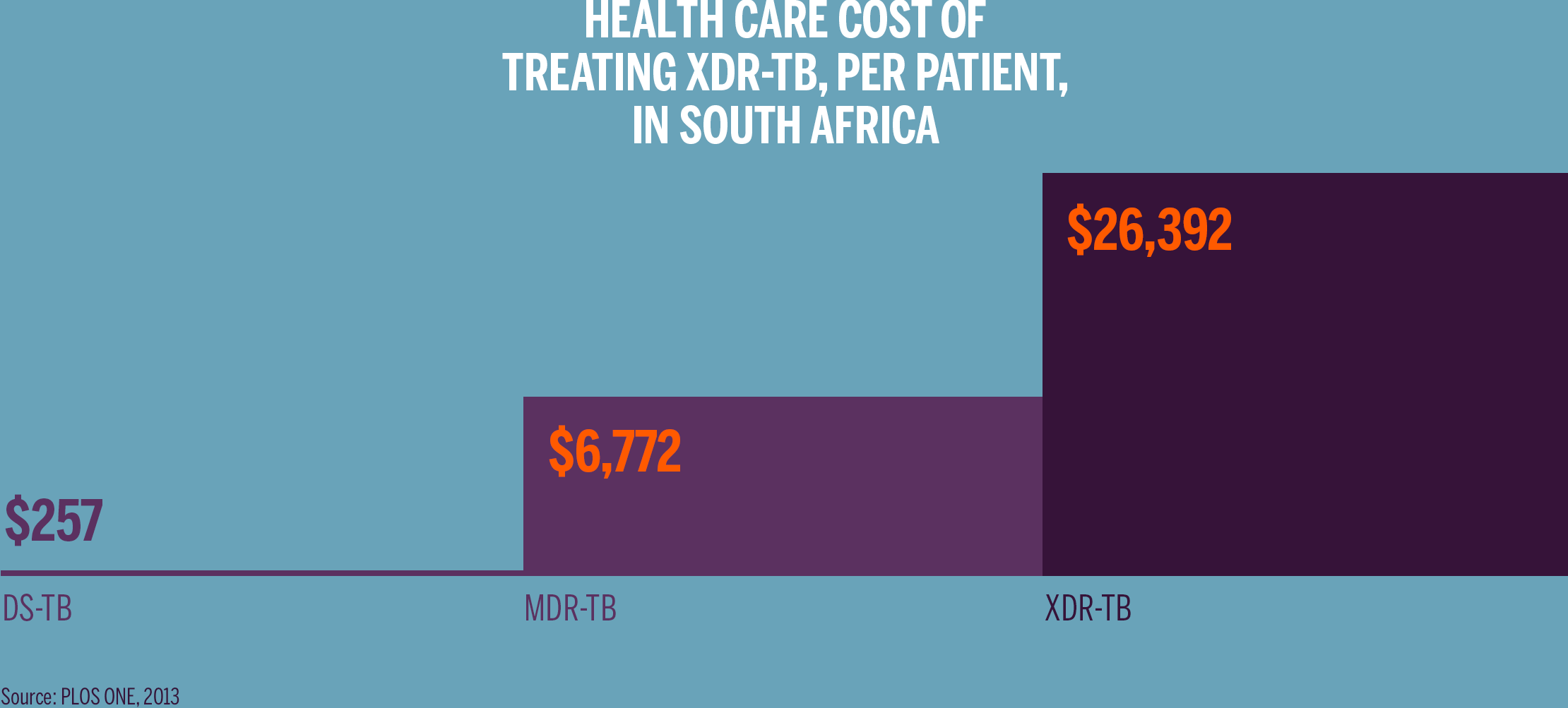
The length of therapy and resources involved render care as exorbitantly expensive. In the United States and Australia, treating a single case of XDR-TB has cost as much as $1 million dollars. In other countries, including high TB burden countries, the actual cost may be less, but is still 100 times as expensive as treatment of drug-sensitive TB. So while XDR-TB may form only a small proportion of the overall TB burden, the cost and lack of efficacy in treating XDR-TB patients contributes disproportionately to the public health threat of TB.

Our vision is to treat the vast majority of patients with active TB with a single ultra-short, simple, and affordable TB regimen that works in virtually all people with tuberculosis. A multi-drug regimen with such broad utility would have a major impact on the global epidemic, saving millions of lives.
The Nix-TB study is a crucial step toward establishing a truly universal
treatment, a regimen to which there is no pre-existing
resistance and could therefore treat any type of TB.
As additional data is generated on the BPaL regimen, TB Alliance will determine whether it is appropriate to pursue the expansion of the BPaL regimen into those with MDR-TB or drug-sensitive TB.
In addition to helping pave the path to a universal regimen for virtually all TB patients, Nix-TB also provides an opportunity to accelerate potential life-saving treatments to patients with limited other options. The hope is it could more immediately help a fairly destitute population stricken with a virtually incurable disease.
