|
Regimen:
PaMZ
|
Stage:
Planning for Phase 3
|

Primary endpoints met in NC-002 trial; planning for a Phase 3 trial of PaMZ.
In NC-002, a clinical trial that was the first of its kind, the novel drug combination PaMZ met its primary endpoint. TB Alliance is now planning for a global Phase 3 registration trial of the PaMZ regimen, consisting of PA-824, moxifloxacin, and pyrazinamide. Data from NC-002 is still under evaluation. The full results of the study will be available in 2014.
The PaMZ regimen has the potential to shorten and simplify treatment for TB and to be compatible with commonly used antiretrovirals. Importantly, the NC-002 study was the first to treat patients with both drug-sensitive and some forms of MDR-TB — a paradigm shift, and potentially a significant advance, in care and management of the disease.

NC-002 not only delivered exciting results, but evidenced TB Alliance's unified development pathway — a development plan designed to accelerate the timeline to deliver impactful novel treatments. As part of this innovative pathway, PaMZ is being tested to both treat drug-sensitive and some forms of drug-resistant disease in a fraction of the time it would otherwise take. By ensuring this regimen undergoes Phase 3 testing, the appropriate data can be collected so that, upon launch, it can achieve uptake and positive impact on public health.
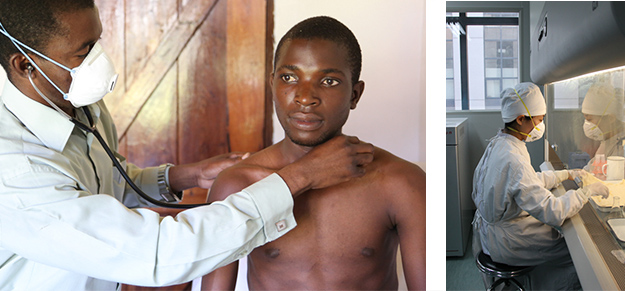
NC-002 was an eight-week Phase 2b clinical trial that enrolled more than 200 patients, and took place at eight sites in South Africa and Tanzania. The trial was conducted based on the TB Alliance's two-week New Combination 1 trial, or NC-001, completed in 2011, which was the first study to test novel TB drugs in combination. Importantly, the NC-002 trial advanced the technical and human infrastructure to conduct TB clinical development. The study relied on collecting information from multiple labs, and also supported Community Engagement work, significantly scaling up global research capacity.