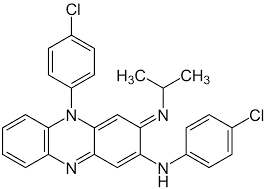
Clofazimine
Clofazimine was originally introduced in 1969 for the treatment of leprosy. The WHO recommendeds it as a second-line compound for use in combination with other drugs for the treatment of drug-resistant tuberculosis.
The mode of action of clofazimine is not defined. Studies have implicated membrane perturbations in Staphylococcus aureus, inhibition of phospholipase A24, and effects on potassium transporters. Clofazamine has a high redox potential and may result in generation of hydrogen peroxide. Transcriptional analysis demonstrated that clofazamine clustered with known respiratory modulators such as phenothiazines, cyanide and azide. This indicates that it may inhibit bacterial cell growth by interfering with electron transport and ATP synthesis.
Common side effects of clofazimine include abdominal and epigastric pain, diarrhoea, nausea, vomiting, and gastrointestinal intolerance. However, perhaps the side effect that most challenges clofazimine’s potential as a TB drug is a reddish-black skin discoloration, which is reversible, but can take months or years to revert. This side effect has been linked to depression and is especially problematic given the stigma TB patients face around the world. TB Alliance efforts to work with clofazimine and additional riminophenazine analogs strive to optimize the compound, thereby reducing these side effects.